Dalton Trans., 2023,52, 5119-5126, https://doi.org/10.1039/D2DT04067K
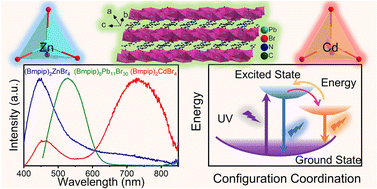
Organic–inorganic hybrid metal halides have been extensively studied because of their great potential in optoelectronics. Herein, we report three hybrid metal halides (Bmpip)2ZnBr4, (Bmpip)2CdBr4, and (Bmpip)8Pb11Br30 (where Bmpip+ is 1-butyl-1-methyl-piperidinium, C10H22N+). (Bmpip)2ZnBr4 and (Bmpip)2CdBr4 crystallize in the P21/c space group with zero-dimensional crystal structures with [MBr4]2− (M = Zn, Cd) tetrahedra isolated by Bmpip+. (Bmpip)8Pb11Br30 crystallizes in the triclinic space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
with one-dimensional corrugated chains constructed from face-sharing [PbBr6]4− octahedra. All of the compounds exhibit excellent ambient and thermal stability. Under UV excitation, all three compounds exhibit very broad emissions. Temperature-dependent photoluminescence measurements indicate that the broad emissions of (Bmpip)2ZnBr4 and (Bmpip)2CdBr4 can be attributed to both the organic cations and self-trapped excitons (STEs) and that the emission of (Bmpip)8Pb11Br30 is assigned to STEs. Density functional theory calculations reveal that the three compounds adopt a direct band gap. This work enriches our understanding of the structure types of hybrid metal halides while revealing their diverse emission mechanisms.

