To fabricate white-light-emitting diodes (white LEDs) with high color-rendering index or full light spectrum emission, the discovery of more efficient deep-red emitting phosphor materials is essential. In this paper, we have synthesized a series of Sr2-2xEu2xSi5N8 (0 ≤ x ≤ 1) solid-solution compounds, and have systemically investigated effects of full-range Eu concentration on their luminescence. Their emission band maximum can be largely tuned from 610 to 725 nm by increasing Eu content. Reabsorption at low Eu2+ concentration while both the energy transfer and Stocks shift at high Eu2+ concentration account for this large spectral red-shift. Luminescent thermal quenching performance gets worse with Eu2+ concentration increasing. The compound with x = 0.15 possesses the best crystallinity and the highest luminescence intensity with the peak position around 660 nm, and still maintains 88.5% room-temperature intensity at 400 K, indicating that great potential for the application as a deep-red phosphor.
Effects of full-range Eu concentration on Sr2-2xEu2xSi5N8 phosphors: A deep-red emission and luminescent thermal quenching
To fabricate white-light-emitting diodes (white LEDs) with high color-rendering index or full light spectrum emission, the discovery of more efficient deep-red emitting phosphor materials is essential. In this paper, we have synthesized a series of Sr2-2xEu2xSi5N8 (0 ≤ x ≤ 1) solid-solution compounds, and have systemically investigated effects of full-range Eu concentration on their luminescence. Their emission band maximum can be largely tuned from 610 to 725 nm by increasing Eu content. Reabsorption at low Eu2+ concentration while both the energy transfer and Stocks shift at high Eu2+ concentration account for this large spectral red-shift. Luminescent thermal quenching performance gets worse with Eu2+ concentration increasing. The compound with x = 0.15 possesses the best crystallinity and the highest luminescence intensity with the peak position around 660 nm, and still maintains 88.5% room-temperature intensity at 400 K, indicating that great potential for the application as a deep-red phosphor.
Effect of polyhedron deformation on the 5d energy level of Ce3+ in lanthanide aluminum perovskites
Physical Chemistry Chemical Physics, January 2019, 21(5)
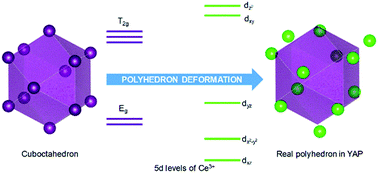
The crystal-field levels of Ce3+ in a series of lanthanide aluminum perovskites have been investigated with reference to polyhedron deformation. For each compound, the corresponding ideal cuboctahedron is derived through a least-square procedure. The virtual energy levels of Ce3+ in these ideal polyhedrons are then obtained considering both crystal-field splitting and spin–orbit coupling. From comparison to real levels, we have a clear understanding of how polyhedron deformation affects the energy levels of Ce3+ in the perovskites.
Tuning of Photoluminescence and Local Structures of Substituted Cations in xSr2Ca(PO4)2–(1 – x)Ca10Li(PO4)7:Eu2+ Phosphors
Chem. Mater., 2017, 29, 3, 1430–1438. https://doi.org/10.1021/acs.chemmater.7b00006

Local structure modification in solid solution is an essential part of photoluminescence tuning of rare earth doped solid state phosphors. Herein we report a new solid solution phosphor of Eu2+-doped xSr2Ca(PO4)2–(1–x)Ca10Li(PO4)7 (0 ≤ x ≤ 1), which share the same β-Ca3(PO4)2 type structure in the full composition range. Depending on the x parameter variation in xSr2Ca(PO4)2–(1 – x)Ca10Li(PO4)7:Eu2+, the vacancies generated in the M(4) site enable the nonlinear variation of cell parameters and volume, and this increases the magnitude of M(4)O6 polyhedra distortion. The local structure modulation around the Eu2+ ions causes different luminescent behaviors of the two-peak emission and induces the photoluminescence tuning. The shift of the emission peaks in the solid solution phosphors with different compositions has been discussed. It remains invariable at x ≤ 0.5, but the red-shift is observed at x > 0.5 which is attributed to combined effect of the crystal field splitting, Stokes shift, and energy transfer between Eu2+ ions. The temperature-dependent luminescence measurements are also performed, and it is shown that the photoionization process is responsible for the quenching effect.
Probing Eu2+ Luminescence from Different Crystallographic Sites in Ca10M(PO4)7:Eu2+ (M = Li, Na, and K) with β-Ca3(PO4)2-Type Structure
Chem. Mater., 2017, 29, 17, 7563–7570. https://doi.org/10.1021/acs.chemmater.7b02724

Eu2+ local environments in various crystallographic sites enable the different distributions of the emission and excitation energies and then realize the photoluminescence tuning of the Eu2+ doped solid state phosphors. Herein we report the Eu2+-doped Ca10M(PO4)7 (M = Li, Na, and K) phosphors with β-Ca3(PO4)2-type structure, in which there are five cation crystallographic sites, and the phosphors show a color tuning from bluish-violet to blue and yellow with the variation of M ions. The difference in decay rate monitored at selected wavelengths is related to multiple luminescent centers in Ca10M(PO4)7:Eu2+, and the occupied rates of Eu2+ in Ca(1), Ca(2), Ca(3), Na(4), and Ca(5) sites from Rietveld refinements using synchrotron power diffraction data confirm that Eu2+ enters into four cation sites except for Ca(5). Since the average bond lengths d(Ca–O) remain invariable in the Ca10M(PO4)7:Eu2+, the drastic changes of bond lengths d(M–O) and Eu2+ emission depending on the variation from Li to Na and K can provide insight into the distribution of Eu2+ ions. It is found that the emission band at 410 nm is ascribed to the occupation of Eu2+ in the Ca(1), Ca(2), and Ca(3) sites with similar local environments, while the long-wavelength band (466 or 511 nm) is attributed to Eu2+ at the M(4) site (M = Na and K). We show that the crystal-site engineering approach discussed herein can be applied to probe the luminescence of the dopants and provide a new method for photoluminescence tuning.
Luminescence Tuning, Thermal Quenching, and Electronic Structure of Narrow-Band Red-Emitting Nitride Phosphors applications
Inorg. Chem., 2017, 56, 19, 11837–11844. https://doi.org/10.1021/acs.inorgchem.7b01816

Exploring high-performance narrow-band red-emitting phosphor is an important challenge for improving white light LEDs. Here, on the basis of three interesting nitride phosphors with similar vierer rings framework structure, two phosphor series, Eu2+-doped Sr(LiAl)1–xMg2xAl2N4 and Sr(LiAl3)1–y(Mg3Si)yN4 (x, y = 0–1), are successfully synthesized by a solid state reaction. They show narrow-band red emission with tunable emission peaks from 614 to 658 nm and 607 to 663 nm. The varying luminescence behaviors with composition and structure are discussed based on centroid shift, crystal field splitting and Stokes shift. On the basis of experimental data, we construct the host referred binding energy (HRBE) and vacuum referred binding energy (VRBE) schemes of divalent/trivalent lanthanide-doped end-member compounds, and further give thermal quenching mechanism of these series phosphors.
Ce3+-Doped garnet phosphors: composition modification, luminescence properties and applications
Chem. Soc. Rev., 2017,46, 275-299. https://doi.org/10.1039/C6CS00551A
Garnets have the general formula of A3B2C3O12 and form a wide range of inorganic compounds, occurring both naturally (gemstones) and synthetically. Their physical and chemical properties are closely related to the structure and composition. In particular, Ce3+-doped garnet phosphors have a long history and are widely applied, ranging from flying spot cameras, lasers and phosphors in fluorescent tubes to more recent applications in white light LEDs, as afterglow materials and scintillators for medical imaging. Garnet phosphors are unique in their tunability of the luminescence properties through variations in the {A}, [B] and (C) cation sublattice. The flexibility in phosphor composition and the tunable luminescence properties rely on design and synthesis strategies for new garnet compositions with tailor-made luminescence properties. It is the aim of this review to discuss the variation in luminescence properties of Ce3+-doped garnet materials in relation to the applications. This review will provide insight into the relation between crystal chemistry and luminescence for the important class of Ce3+-doped garnet phosphors. It will summarize previous research on the structural design and optical properties of garnet phosphors and also discuss future research opportunities in this field.
After-glow, luminescent thermal quenching, and energy band structure of Ce-doped yttrium aluminum-gallium garnets
Yttrium aluminum-gallium garnets with cerium doped is most widely used as green-yellow phosphor in solid state lighting. Extensive research has been performed on this material concerning the luminescent thermal quenching resistance and persistent luminescence. In this paper we find that a negative correlation exists between temperature-dependent luminescence and persistent luminescence with gallium content varying. The correlation originates from the electronic structures which influence both the thermal quenching of luminescence and persistent luminescence. A detailed crystal-field calculation has been performed to understand the peak shifts. In addition, theoretical calculations reveal that oxygen vacancies provide trap levels which implement the persistent luminescence. This material could be used as potential blue-light excited persistent luminescent material, with the after-glow time up to about 1. h with only cerium as the dopant, which is expected to be prolonged by co-doping other elements. This work may be helpful in guiding the discovery of other after-glow materials.
Crystal Structure and Photoluminescence Evolution of La5(Si2+xB1–x)(O13–xNx):Ce3+ Solid Solution Phosphors
J. Phys. Chem. C., 2015, 119, 17, 9488. https://pubs.acs.org/doi/10.1021/acs.jpcc.5b01211

A series of iso-structural La5(Si2+xB1–x)(O13–xNx):Ce3+ phosphors with apatite structure have been prepared. A combination of powder X-ray diffraction and neutron scattering technique was employed to explore the crystal structural evolution and the rigid nature from oxy- to oxynitride-based apatites, and some local structures were also characterized by HRTEM and 29Si NMR data, respectively. The new La5(Si2+xB1–x)(O13–xNx):Ce3+ solid solution phosphors gave continuously controlled emission from 421 nm [La5Si2BO13:Ce3+, end-member (x = 0)] to 463 nm (La5Si3O12N:Ce3+, end-member (x = 1)). Substitution of B3+ and O2– by Si4+ and N3– in La5(Si2+xB1–x)(O13–xNx):Ce3+ phosphors produced more covalency into the crystal field environment around the Ce3+ ions inducing the red-shifted emission, further improving the thermal stability of the oxynitride-based apatite phosphors. The proposed approach from oxy- to oxynitride based iso-structural phases could significantly contribute to future research in designing complex solid solution phosphors.
Complementary method to locate atomic coordinates by combined searching method of structure-sensitive indexes based on bond valence method
Chin. Phys. B., 2015, 24, 106101. https://iopscience.iop.org/article/10.1088/1674-1056/24/10/106101

Bond valence method illustrates the relation between valence and length of a particular bond type. This theory has been used to predict structure information, but the effect is very limited. In this paper, two indexes, i.e., global instability index (GII) and bond strain index (BSI), are adopted as a judgment of a search-match program for prediction. The results show that with GII and BSI combined as judgment, the predicted atom positions are very close to real ones. The mechanism and validity of this searching program are also discussed. The GII & BSI distribution contour map reveals that the predicted function is a reflection of exponential feature of bond valence formula. This combined searching method may be integrated with other structure-determination method, and may be helpful in refining and testifying light atom positions.

